 |
 |
AbstractSwitch/sucrose non-fermentable-related matrix-associated actin-dependent regulator of chromatin subfamily B member 1 (SMARCB1)-deficient sinonasal carcinoma is a rare subtype of sinonasal undifferentiated carcinoma (SNUC). Due to the highly aggressive nature of the disease course, it is important to diagnose early and use multimodal treatments in the course of the disease. Two main morphologic patterns are commonly identified under the microscope: plasmacytoid/rhabdoid and basaloid. Furthermore, it demonstrates a total absence of integrase interactor 1 expression upon immunohistochemical staining. Here, we present two cases of SMARCB1-deficient sinonasal carcinoma with contrasting outcomes.
IntroductionOne patient per 100000 persons is diagnosed with a malignant tumor in the sinonasal tract each year worldwide, accounting for 5% of all head and neck cancers [1]. Although squamous cell carcinoma and adenocarcinoma are among the most frequently diagnosed disease entities in the sinonasal tract, as defined in the 2017 4th edition of the World Health Organization (WHO) Classification of Head and Neck Tumors [2], relatively new pathologies have been reported. These pathologies which were conventionally reported as sinonasal undifferentiated carcinoma (SNUC) include switch/sucrose non-fermentable-related matrix-associated actin-dependent regulator of chromatin subfamily B member 1 (SMARCB1)-deficient sinonasal carcinoma and human papillomavirus (HPV)-related multiphenotypic sinonasal carcinoma [1]. SMARCB1-deficient sinonasal cancer is caused by the mutation in switch/sucrose non-fermentable (SWI/SNF)-related matrix-associated actin-dependent regulator [3]. Due to the lack of distinct signs and symptoms, it is often detected late in the course of the disease, which leads to a poor prognosis [4]. Consequently, making an early diagnosis and using the proper multimodal treatment is crucial.
CaseCase 1A 27-year-old male who had no underlying health condition presented to a tertiary care hospital with a 6-month history of left epistaxis. The CT scan demonstrated soft tissue density at the left ethmoid sinus, septal deviation to the left, and both inferior turbinate hypertrophy. The patient received an endoscopic sinus surgery (ESS) and the pathology report from the ESS revealed squamous cell carcinoma, poorly differentiated. The patient was referred to our hospital. The CT taken at our hospital revealed a soft tissue signal in the left ethmoid sinus and nasal cavity (Fig. 1A-C). A MR T2-weighted imaging showed an intermediate to low signal intensity at the medial and superoposterior aspect of the left ethmoid sinus, and the medial aspect of the left nasal cavity (Fig. 1D and E). No evidence of neck lymphadenopathy was found upon imaging work-up. There were no signs of intracranial and intraorbital extension. The diagnosis was SMARCB1-deficient sinonasal carcinoma (cT2N0M0) according to our formal pathology report based on the slides transferred from the prior hospital. The patient underwent left endoscopic endonasal resection. The final pathology report after surgery confirmed SMARCB1-deficient sinonasal carcinoma (pT2N0M0). On histopathologic examination, the tumor was infiltrating the subepithelial stroma, showing a solid sheet-like growth pattern. The tumor was composed of epithelioid to spindle cells, with scanty cytoplasm. On immunohistochemical staining, the tumor cells were positive for cytokeratin and showed complete loss of integrase interactor 1 (INI-1) expression. As a result, the tumor was diagnosed as SMARCB1-deficient sinonasal carcinoma (Fig. 2). The patient was discharged on postoperative day 4. The endoscopic exam at the outpatient clinic showed well-healed mucosa without any signs of complication or tumor recurrence on postoperative month 2. Postoperative concurrent chemoradiation therapy was provided for the following reasons; the patient was young and had no underlying diseases. SMARCB1-deficient sinonasal carcinoma is known for its poor prognosis, which necessitates multimodal treatment approaches. Additionally, there was an enlargement of the left retropharyngeal lymph node, which was not removed during the surgery. Fourty five Gy of radiation treatment over the course of 25 days was given with a daily fraction of 1.8 Gy postoperatively with 2 cycles of weekly cisplatin (cisplatin 40 g/m2). The local tumor bed was then given a 14 Gy local boost with a daily fraction of 2 Gy over the course of seven days, and a 6.6 Gy local extra boost with a daily fraction of 2.2 Gy over the course of three days. The MRI showed no evidence of residual or recurrent tumor at 14 months after the surgery.
Case 2An 88-year-old male who had undergone prostate cancer surgery and had underlying hypertension arrived at a tertiary care hospital with a 2-month history of both nasal obstructions. The endoscopic intranasal examination revealed a polypoid mass in the right nasal cavity. The MR showed a T2 intermediate signal intensity mass in the right nasal cavity and ethmoid sinus with extension to the right maxillary sinus and choana (Fig. 3A and B). The CT revealed an enhancing solid mass involving the right nasal cavity and ethmoid sinus with the erosion of the right inferior orbital wall, the extent to the right nasolacrimal duct & sac, and left ethmoid sinus, and no definite intracranial extension (Fig. 3C and D). The CT also demonstrated necrotic lymph nodes at left level II and right neck levels II and V (Fig. 3E). The final diagnosis was SMARCB1-deficient sinonasal carcinoma (cT4aN2cM0) and underwent right radical maxillectomy along with right total parotidectomy and both modified radical neck dissection. The pathologistвҖҷs report after the surgery confirmed SMARCB1-deficient sinonasal carcinoma with the invasion of the right maxilla bone, nasolacrimal duct, and orbit along with both neck lymph node metastasis and extranodal extension (pT4aN3bM0). On histopathologic examination, the tumor was infiltrating subepithelial stroma. The tumor was composed of rhabdoid cells, with large eccentric nuclei and abundant eosinophilic cytoplasm. On immunohistochemical staining, the tumor cells showed a complete loss of INI-1 expression (Fig. 4). At postoperative month 3, the patient complained of right cheek swelling. Subsequently, the MR revealed T2 intermediate, enhancing soft tissue lesions in the medial canthal area and cheek subcutaneous layer. The core needle biopsy report confirmed metastatic SMARCB1-deficient sinonasal carcinoma. The PET-CT revealed multiple distant metastases in the lung and the patient was referred to hospice care.
DiscussionSince the 1st cases of SMARCB1-deficient sinonasal carcinoma were reported by two independent groups [3,4], Several case series have been published in regard to SMARCB1-deficient sinonasal carcinoma, including the largest case series reported in 2017 [4,5]. However, we suspect there would be more cases of SMARCB1-deficient sinonasal carcinoma worldwide than already reported, considering that SMARCB1-deficient sinonasal carcinoma is relatively new entity that has been recognized after the recent development of new laboratory technology to find SMARCB1 tumor suppressor gene deletion or mutation [6]. SMARCB1-deficient sinonasal carcinoma is a highly aggressive tumor that leads to poor prognosis and there currently is no targeted treatment for this disease. Therefore, it is critical to recognize and diagnose SMARCB1-deficient sinonasal carcinoma early in its disease course. Our case report presents 2 cases of SMARCB1-deficient sinonasal carcinoma with contrasting outcomes.
SMARCB1-deficient sinonasal carcinoma is recognized for its aggressively invasive behavior, often involving multiple paranasal sinuses [7]. Under the microscope, two main morphologic patterns are frequently observed: plasmacytoid/rhabdoid and basaloid. The plasmacytoid/rhabdoid variant is characterized by the presence of plasmacytoid/rhabdoid cells arranged in sheets, with prominent eosinophilic cytoplasm and eccentric nuclei. In contrast, the basaloid variant consists of basaloid cells forming nests and lobules with a high nuclear cytoplasmic ratio, exhibiting peripheral palisading. Additionally, there are scattered plasmacytoid/rhabdoid cells in the basaloid variant [1].
SMARCB1-deficient sinonasal carcinoma predominantly affects males and spans a broad age range from 16 to 88 years (mean age of 51 years), demonstrating a preference for the ethmoid sinus and nasal cavity. Typically, the disease follows an aggressive clinical course, with the majority of cases presenting as advanced stages such as pT3/T4, often resulting in frequent recurrence and patient mortality (Table 1) [7-12]. Histologically, the tumor presents as clusters or branching structures comprising uniform blue cells characterized by limited cytoplasm, a high ratio of nucleus to cytoplasm, and prominent nucleoli within a fibrous stroma. No signs of abnormal growth on the surface are apparent; however, occasional tumor cells infiltrate the lining in a pagetoid pattern. Tumor cells exhibit a tendency to form palisades, accompanied by abundant cytoplasmic vacuoles and the absence of squamous or glandular differentiation. Another variant displays the proliferation of plasmacytoid cells with eccentric nuclei and hyaline eosinophilic cytoplasm. Brisk cell division is frequently observed, accompanied by extensive necrosis [1,4,5]. Tumor cells typically exhibit positive immunoreactivity for pan-cytokeratin, while P63, CK5, and CK7 are positive in approximately half of the cases. Other markers, including synaptophysin, chromogranin, CD56, CD117, and P16, may exhibit limited positivity. However, the tumor is negative for HPV, as confirmed by in situ hybridization. In situ hybridization for Epstein Barr virus and immunohistochemistry for nuclear protein in testis also yield negative results in tumor cells. A notable characteristic feature is the absence of SMARCB1 (INI1) expression in tumor cells [1].
According to the WHO, SNUC is an extremely aggressive type of carcinoma that does not exhibit squamous or glandular characteristics. It often spreads to areas beyond the sinuses, such as the orbit and skull base. Typically, treatment for SNUC involves a combination of surgical procedures and nonsurgical approaches like chemotherapy and/or radiation therapy. Unfortunately, regardless of the chosen treatment, SNUC has a poor prognosis, with a significant likelihood of recurrence and extremely low survival rates [13]. Additionally, the absence of SMARCB1 expression is associated with an overall poorer prognosis [14].
In summary, we presented 2 cases of poorly differentiated sinonasal carcinoma demonstrating SMARCB1 deficiency. Although it is a highly aggressive entity, with early stage diagnose of the disease, the prognosis may be good as in our 1st case. It is essential to have newly emerging sinonasal carcinoma such as SMARCB1-deficient sinonasal carcinoma in the list of differential diagnoses when one encounters poorly differentiated sinonasal carcinoma.
ACKNOWLEDGMENTSThis case report was approved as exempt and the waiver of patient consent was obtained from the Institutional Review Board of Asan Medical Center (Exemption number S2023-0989-0001).
NotesAuthor Contribution Conceptualization: Myeong Sang Yu. Data curation: Taegyeong Kim, Won-Gi Hong. Formal analysis: Taegyeong Kim. Investigation: Taegyeong Kim. Methodology: Taegyeong Kim, Myeong Sang Yu. Project administration: Taegyeong Kim. Resources: Myeong Sang Yu. Supervision: Myeong Sang Yu, Kyung-Ja Cho. Validation: Myeong Sang Yu. Visualization: Myeong Sang Yu. WritingвҖ”original draft: Taegyeong Kim. WritingвҖ”review & editing: Taegyeong Kim, Myeong Sang Yu. Fig.В 1.Preoperative CT findings. A-C: CT revealed soft tissue signal in the left ethmoid sinus and nasal cavity (circle). D and E: MRI revealed an intermediate to low signal intensity at medial and superoposterior aspect of the left ethmoid sinus, and medial aspect of the left nasal cavity (arrows). 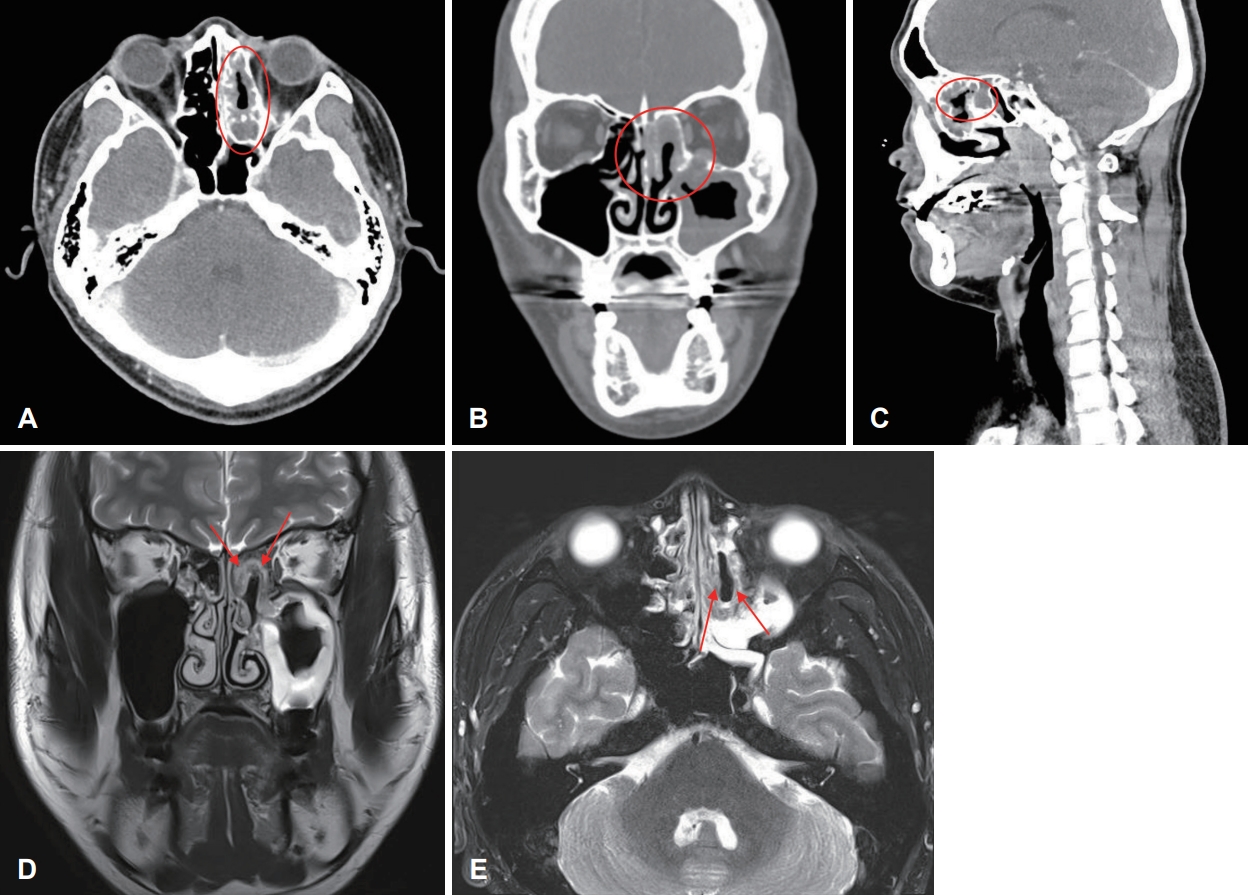
Fig.В 2.Histopathologic findings. A: The tumor cells are arranged in solid sheets, and infiltrate subepithelial stroma in the biopsy specimen (hematoxylin and eosin [H&E], Г—50). B: The tumor is composed of epithelioid to spindle cells (H&E, Г—400). C-E: Tumor cells show positivity for cytokeratin (immunohistochemical staining, Г—200; C), positivity for p63 (immunohistochemical staining, Г—200; D), and complete loss of INI-1 in comparison to positive stromal cells (immunohistochemical staining, Г—200; E). F: A few foci of tumor were notified in surgical specimen, also showing solid sheet-like arrangement of epithelioid to spindle cells (H&E, Г—200). 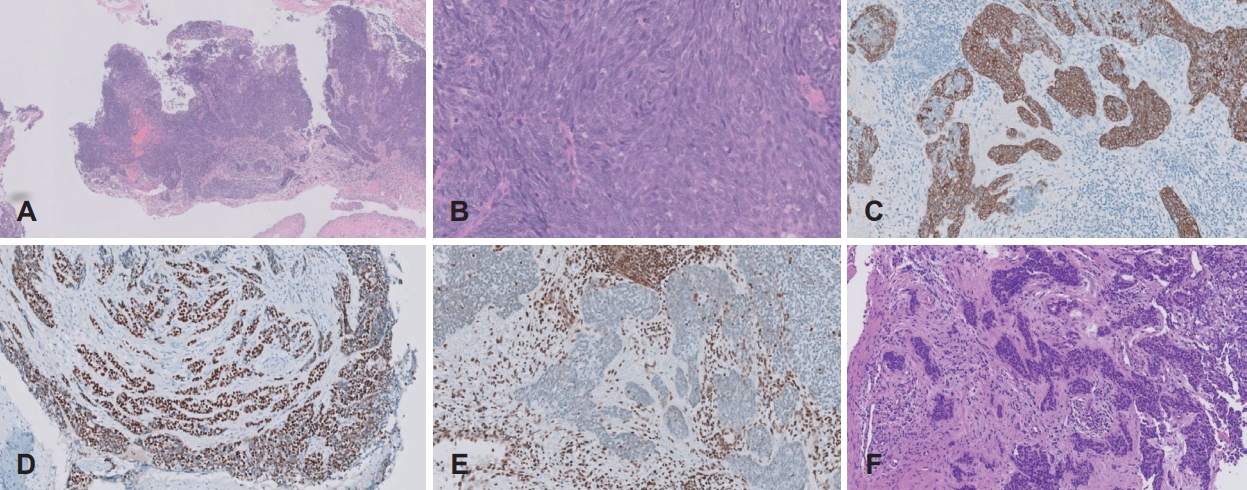
Fig.В 3.Preoperative image findings. A and B: MR images showing T2 intermediate signal intensity mass in the right nasal cavity and ethmoid sinus with extension to the right maxillary sinus and choana. C: CT images showing enhancing sold mass involving the right nasal cavity and ethmoid sinus with erosion of the right inferior orbital wall. D: The extent to the right nasolacrimal duct & sac, left ethmoid sinus. E: Necrotic lymph nodes at both level II. 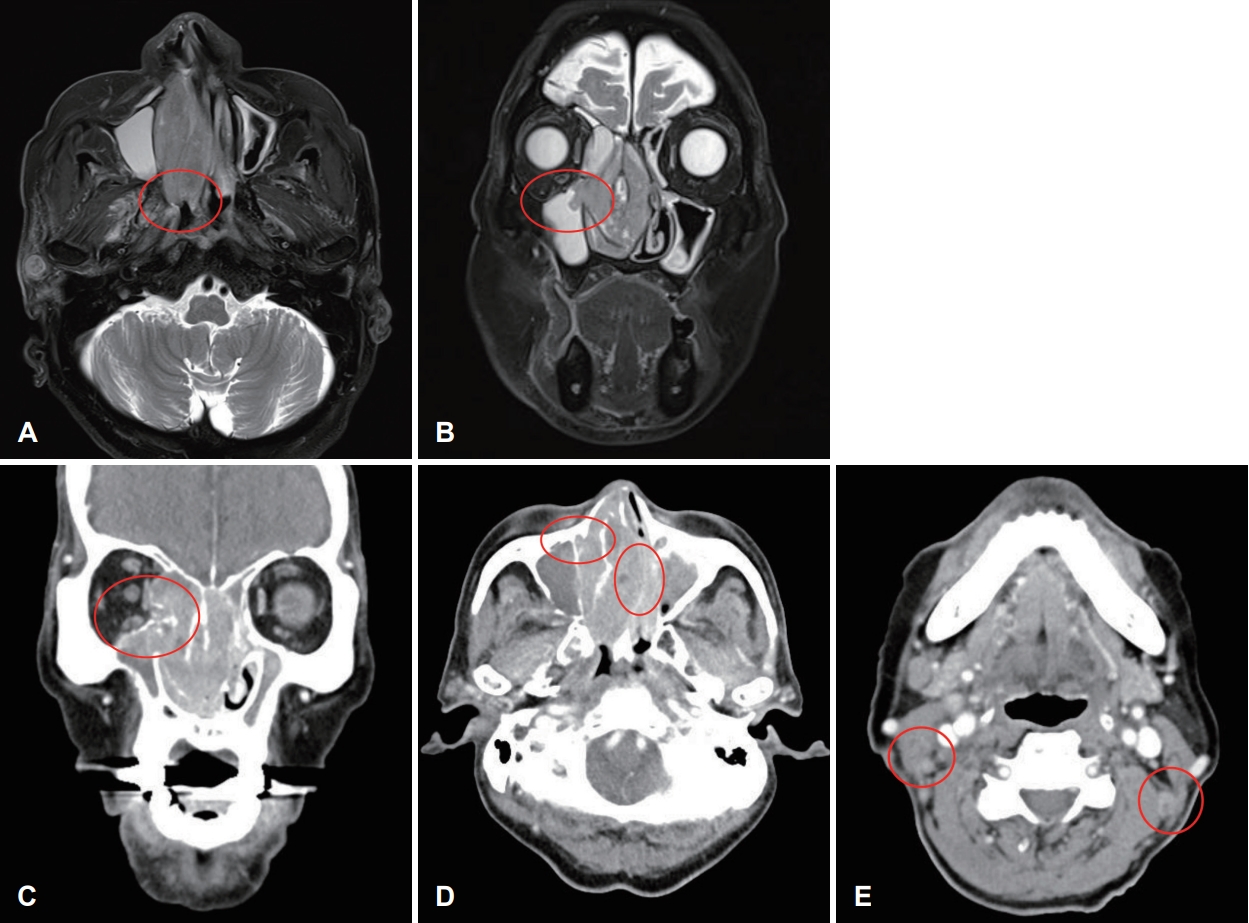
Fig.В 4.Histopathologic findings. A: The tumor is infiltrating subepithelial stroma in biopsy specimen (hematoxylin and eosin [H&E], Г—100). B: Tumor cells show rhabdoid features, with large eccentric nuclei with abundant eosinophilic cytoplasm (H&E, Г—400). C: Tumor cells show loss of INI-1 expression in comparison with positive stromal cells (immunohistochemical staining, Г—200) D: Lobulated sheet-like proliferation of tumor cells in surgical specimen (H&E, Г—20). E: Tumor cells in surgical specimen also show rhabdoid features (H&E, Г—100). 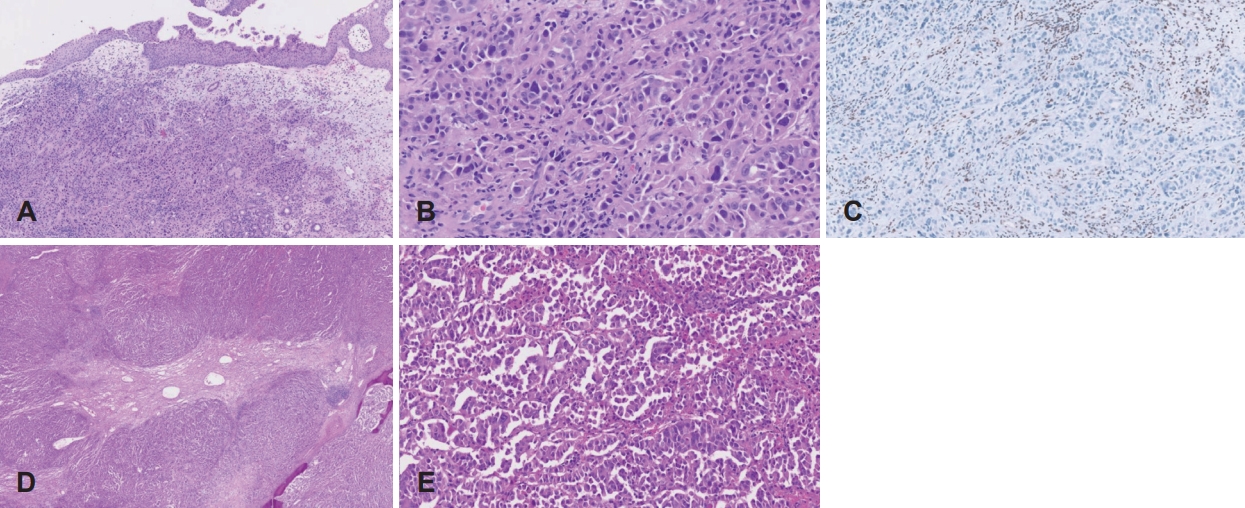
TableВ 1.Clinical features of SMARCB1-deficient sinonasal carcinoma from the current and past case reports
SMARCB1, switch/sucrose non-fermentable-related matrix-associated actin-dependent regulator of chromatin subfamily B member 1; M, male; F, female; PD, poorly differentiated; SCC, squamous cell carcinoma; SNUC, sinonasal undifferentiated carcinoma; NA, not available; CCRT, concurrent chemoradiation therapy; CTx, chemotherapy; XRT, external beam radiotherapy; NED, no evidence of disease; AWD, alive with disease; DOD, died of disease REFERENCES1. Agaimy A, Hartmann A, Antonescu CR, Chiosea SI, El-Mofty SK, Geddert H, et al. SMARCB1 (INI-1)-deficient sinonasal carcinoma: A series of 39 cases expanding the morphologic and clinicopathologic spectrum of a recently described entity. Am J Surg Pathol 2017;41(4):458-71.
2. Thompson L. World Health Organization classification of tumours: pathology and genetics of head and neck tumours. Ear Nose Throat J 2006;85(2):74.
3. Bishop JA, Antonescu CR, Westra WH. SMARCB1 (INI-1)-deficient carcinomas of the sinonasal tract. Am J Surg Pathol 2014;38(9):1282-9.
4. Agaimy A, Koch M, Lell M, Semrau S, Dudek W, Wachter DL, et al. SMARCB1(INI1)-deficient sinonasal basaloid carcinoma: A novel member of the expanding family of SMARCB1-deficient neoplasms. Am J Surg Pathol 2014;38(9):1274-81.
5. Bishop JA, Antonescu CR, Westra WH. SMARCB1 (INI-1)-deficient carcinomas of the sinonasal tract. Am J Surg Pathol 2014;38(9):1282-9.
6. Yang H, Zhou L, Zhong G, Li X, Wang Y. SMARCB1 (INI-1)-deficient sinonasal carcinoma: A case report and literature review. Ear Nose Throat J In press. 2022.
7. Wasserman JK, Dickson BC, Perez-Ordonez B, de Almeida JR, Irish JC, Weinreb I. INI1 (SMARCB1)-deficient sinonasal carcinoma: A clinicopathologic report of 2 cases. Head Neck Pathol 2017;11(2):256-61.
8. Barresi V, Branca G, Raso A, Mascelli S, Caffo M, Tuccari G. Atypical teratoid rhabdoid tumor involving the nasal cavities and anterior skull base. Neuropathology 2016;36(3):283-9.
9. Bell D, Hanna EY, Agaimy A, Weissferdt A. Reappraisal of sinonasal undifferentiated carcinoma: SMARCB1 (INI1)-deficient sinonasal carcinoma: A single-institution experience. Virchows Arch 2015;467(6):649-56.
10. Jamshidi F, Pleasance E, Li Y, Shen Y, Kasaian K, Corbett R, et al. Diagnostic value of next-generation sequencing in an unusual sphenoid tumor. Oncologist 2014;19(6):623-30.
11. Shatzkes DR, Ginsberg LE, Wong M, Aiken AH, Branstetter BF 4th, Michel MA, et al. Imaging appearance of SMARCB1 (INI1)-deficient sinonasal carcinoma: A newly described sinonasal malignancy. AJNR Am J Neuroradiol 2016;37(10):1925-9.
12. Zeng M, Chen C, Yang S, Chen X. SMARCB1 (INI1)-deficient sinonasal carcinoma: A newly described entity. Int J Clin Exp Pathol 2016;9(3):3454-8.
|
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||

 |
 |