 |
 |
AbstractBackground and ObjectivesThis study investigates the role of manuka honey in the healing of postoperative mastoid cavity.
Subjects and MethodThis was a single centre prospective study on 40 consecutive patients of chronic otitis media undergoing canal wall down mastoidectomy. Manuka honey soaked in gel foam was kept in the mastoid cavity for the study group and antibiotic soaked gel foam was kept for the control group. Culture swabs from mastoid granulations were sent at various times from both groups. The healing of the mastoid cavity was assessed in the follow up period.
ResultsPreoperatively 15 out of 20 patients (75%) had a positive aural swab culture in the study group while 11 out of 20 (55%) in the control group had a positive aural swab culture. The most common organism isolated was Pseudomonas aeruginosa and Proteus mirabilis. One month after mastoidectomy only 4 patients (20%) had sterile culture and 16 patients (80%) had grown organisms; in the control group, 7 patients (35%) had sterile culture and 13 patients (65%) had growth on culture. The mean merchant scores for the study group and the control were 2.61 (2-5) and 2.05 (1-4), respectively. At 3 months 13 patients (65%) with sterile culture and 7 patients (35%) had growth on culture; in the control group, 16 patients (80%) had sterile culture and 4 had shown persistent growth on culture (p=0.28). All positive cultures were aerobic in both groups. The mean merchant scores for the study group and the control were 1.03 (0-4) and 0.7 (0-3), respectively (p=0.09).
IntroductionPersistent otorrhea and granulations in the mastoid cavity are common post-operative complications of canal wall down mastoidectomy. Microbial contamination is one of the major factors for persistent otorrhea [1]. Albert, et al. [2] did a study to detect the presence of bacteria in mastoid granulations. They found that mastoid granulations contain polymicrobial pathogens and positive cultures were obtained irrespective of aditus patency, age, stage, and duration of disease activity [2].
Both systemic antibiotics and topical antibiotics have been used in the postoperative period to eradicate infection and improve healing of the mastoid cavity [3,4]. However its use in routine mastoid surgery is controversial and there is a risk of antibiotic resistance [5]. Winerman, et al. [6] stated that there was no role of prophylactic or postoperative antibiotics in uncomplicated mastoid surgery. Topical antibiotics can penetrate the inner ear of patients with tympanic membrane perforation and can cause vestibulotoxicity [7]. There is continuous search for adjuvant medical products that would reduce the need for antibiotics in mastoid surgery. On literature search for other alternatives, we found that manuka honey has been used in the treatment of chronically discharging mastoid cavities to make them dry and was found to be safe for use in ear [8].
Manuka honey is obtained from manuka shrub, leptospermum scoparium, aboriginal to South East Australia and New Zealand. It contains methyl glyoxal, D-glucono-Оҙ-lactone, hydrogen peroxide which is responsible for its antibacterial action. It also has antioxidants [9]. Low water content makes honey inhabitable to microbes [10].
Manuka honey has antibacterial properties against Pseudomonas, Methicillin sensitive and methicillin resistant Staphylococcus aureus, and also has activity against biofilms [11,12].
It can obviate the use of systemic antibiotics in mastoid surgery. It has also been shown to prevent the development of antibiotic resistance.
Can manuka honeyвҖҷs antibacterial properties be used for preventing infection and facilitating healing of mastoid cavity?
This study was planned to study the role of manuka honey in the healing of postoperative mastoid cavity and to find whether it can obviate the need of topical and systemic antibiotics after mastoid surgery.
Subjects and MethodThis study was a single centre prospective study and included 40 consecutive cases of active/inactive squamosal chronic otitis media from January 2016 to June 2017. The study was approved by the Institutional Ethics Committee with IRB number NK/2538/MS/835-36. Patients with age <10 years and >60 years, immunocompromised patients, history of antibiotic usage within 7 days prior to surgery and patients with complications of chronic otitis media were excluded from the study.
All the patients underwent a detailed otolaryngological and microscopic examination along with pure tone audiometry. A high resolution CT scan was done in all patients. Plan of surgery was discussed with patients in our otology clinic in all patients. Those planned for canal wall down mastoidectomy were included in the study. Informed consent was taken for participation in the study. Those consenting to participate in the study were randomly assigned into two groups.
Group 1: manuka honey medicated gel foam kept in mastoid cavity.
Group 2: polymyxin B sulphate, neomycin sulphate, and hydrocortisone medicated gel foam in mastoid cavity.
All patients underwent surgery under general/local anaesthesia by a single senior surgeon. Preoperative swab for bacterial culture sensitivity was done in the operation theatre under a microscope following strict asepsis. Canal wall down mastoidectomy with or without ossicular reconstruction was done through a standard post auricular approach. Gel foam soaked in manuka honey (medical grade purchased from online site Amazon.in) was kept in mastoid cavity at the time of surgery in the intervention group. In the control group polymyxin B sulphate, neomycin sulphate, and hydrocortisone medicated gel foam was kept in mastoid cavity.
Manuka honey soaked gel foam was kept in the cavity at 1st and 2nd month postoperatively in the intervention group. Topical antibiotics drops were given in the control group in follow up period. All patients were followed up on: 1st day and weeks 1, 4, 8, and 12 weeks. Mastoid cavity was assessed by an independent observer. On 1st day, examination was done to look for wound site hematoma. On 1st, 4, 8, and 12th week oto-endoscopy was done by independent observers and mastoid cavity condition was assessed using
Statistical analysisThe statistical analysis of various parameters in this study was done as follows.
All parametric data was first subjected to normality test (Kolmogorov Smirnov test) for studying distribution. All the normal data was compared using paired t test and all the skewed data was tested for significance with Mann Whitney U test. All the categorical and classified data was tested at a given point of time with chi-square test. Parametric matched normally distributed data was compared using t test and skewed data was compared by Wilcoxon signed rank test and Mann Whitney U test. Non-parametric data was compared using Wilcoxon signed rank test. Corrections for the confounding factors were done as required during the analysis. The variables for measuring central tendencies and for study of distribution in the analysis of data was mean and standard deviation in case of quantitative data and was median and inter-quartile range in case of qualitative data.
ResultsA total number of 40 patients with attico-antral type of chronic otitis media who fulfilled inclusion and exclusion criteria were enrolled in the study. Out of the 20 patients in the study group, 10 were males and 10 females. In the control group, there were 12 males and 8 females. The mean age was 24.33 years (10-55 yrs) in the study group and 23.25 years (13-65 yrs) in the control group. The data was found to be homogenous with regard to the clinical symptoms, extent of disease, type of disease, and the extent of surgery performed.
Of the 20 patients in the study group, 15 (75%) had a positive growth on preoperative aural swab culture, while 5 (25%) had sterile culture. In the control group, 11 patients (55%) had a positive aural swab and 9 patients (45%) had sterile culture. In the study group, culture showed growth of aerobic bacteria in 12 patients (60%) while 3 (15%) patients had anaerobic growth. In the control group, 10 patients (50%) had aerobic growth while 1 patient had anaerobic growth. The commonest organism isolated was Pseudomonas aeruginosa in 7 out of 20 (35%) patients in the study group and 8 out of 20 (40%) in the control group (Fig. 1).
Post-operatively parameters otorrhea, itching and discomfort were assessed by Visual Analogue Scale scoring at various times of follow up.
The mean otorrhea score at day 1 was 0.33 (0-1.7), 1st month was 4.67 (1-5), 2nd month was 3.33 (1-4), and 3rd month was 1.44 (1-3) in the study group. In the control group it was 0.30 (0-1.5) at day 1, 3.00 (1-5) at 1 month, 1.65 (0-4) at 2nd month, and 0.75 (0-2) at 3 months. We found statistically significant increase in otorrhea at 8th postoperative day (p=0.008), 1st month (p=0.006) and 2nd month (p=0.01).
At 3 months, 10 out of 20 (50%) patients in the study group had persistent otorrhea, of which 5 patients (50%) had sterile culture, 5 patients had growth of organisms on culture. While in the control group, 8 out of 20 (40%) had persistent otorrhea of which only 3 patients had sterile culture.
Mean discomfort score was 0.83 (0-2) on day 1, 1.50 (0-4) at 1 month, 0 at 3 months after surgery in the study group, while in the control group it was 0.0 (0-2) at day 1 and 0 at 3 months. We found statistically significant increase in the discomfort score at day 1 (p=0.03). There was no discomfort experienced by the patients at the end of three months in both the groups.
Mean itching score was also assessed. Mean itching score was found to be 0.33 (0-3) at day 1, 0.72 (0-4) at day 8, 0.50 (0-3) at 1 month, 0.33 (0-2) at 2nd month, and 0.15 (0-1) at 3rd month in the study group, while in the control group it was 0.15 (0-1) at day 1, 1.50 (0-4) at 8 day, 1.20 (0-4) at 1 month, 0.30 (0-2) at 2 month, and 0.15 (0-2) at 3 months. We found slight increase in the itching scores in the initial postoperative period in the study group.
Visual analog scoring of pain was found to be comparable in both the groups although the mean scoring of pain was found to be slightly higher in the manuka honey group (p>0.05). Pain score was higher on day 1 post surgery 3.60 (0-5) in the topical antibiotic group vs. 3.83 (0-5) in the manuka honey group and it gradually decreased to less than 1 at 3 months postoperative period in both the groups (Fig. 2).
Post-operative mastoid cavity culture at day 8 showed that 17 out of 20 (85%) had grown organism and only 3 patients had sterile cultures in the study group. While in the control group, 9 out of 20 (45%) showed growth on culture and 11 (55%) had sterile cultures. Out of 17 patients in the study group with positive culture, 11 had growth of aerobes and 6 patients had combined aerobic and anaerobic growth. While in the control group out of 9 patients with positive culture, 8 patients had aerobic growth and 1 had fungal growth (Figs. 3 and 4).
We compared the preoperative aural swab cultures with that of day 8 cultures in both the groups. Out of 5 patients who had sterile cultures preoperatively in the study group, only one of them remained sterile while 4 patients developed growth. Fifteen patients had growth on culture preoperatively in the study group, 13 of them continued to have growth and 2 became sterile. While in the control group 11 patients who had positive cultures preoperatively, 7 of them continued to have growth whereas 4 turned sterile (Figs. 3 and 4).
At 1 month, only 4 (20%) patients had sterile culture while 16 patients had grown organisms in the study group. Thirteen patients had aerobic and 3 patients had combined aerobic and anaerobic infection. While in the control group 7 out of 20 (35%) had sterile culture and 13 (65%) had growth on culture and all were aerobic.
At 2nd month, 7 (35%) patients had sterile culture while 13 patients had growth on culture in the study group. Out of the 13 patients, 12 had aerobic growth and 1 patient had combined aerobic and anaerobic growth. In the control group 10 out of 20 (50%) patients had sterile cultures and 10 patients (50%) had growth on culture and all of them were aerobic.
At 3 months, 13 patients (65%) had sterile cultures while 7 patients continued to show growth in the study group. Out of these 7 patients who had positive culture, 5 had grown pseudomonas and 2 had proteus. While in the control group, 16 patients (80%) had sterile culture and 4 of them showed growth on culture and all 4 grew Pseudomonas.
On comparing culture results between 2nd month and 3rd month, we found that, in the study group, 7 patients who had sterile cultures at 2nd month continued to remain sterile at 3rd month. While out of 13 patients who had growth at 2nd month, 6 became sterile and 7 continued to have growth. One patient who had anaerobic infection at 2nd month in the study group had clearance of anaerobic infection at 3rd month (Figs. 3 and 4). In the control group, 10 patients who had sterile cultures at 2nd month continued to remain sterile at 3rd month. While out of 10 patients who had growth at 2nd month, 6 became sterile and 4 continued to have growth even at the end of 3rd month.
Adapted mean merchant score was used to assess the mastoid cavity status. The mean merchant score at day 8 was 1.50, at 1st month was 2.61, at 2nd month was 2.39, at 3rd month was 1.39 in the study group. While in the control group, it was 1.15 at day 8, 2.05 at 1st month, 1.25 at 2nd month, and 0.70 at 3rd month. There was a statistically significant difference in the inflammation score at 2nd month between both the groups (p=0.01). This signifies the reduction in inflammation, granulation tissue formation and healing of the mastoid cavity with passage of time (Table 1).
Ten out of 20 patients (50%) had dry cavity at the end of 3 months in the study group. Of the 10 discharging cavities, 5 were sterile and 5 showed growth on culture. Of the 5 sterile cultures, 3 became dry after 2 months of further follow up. Thirteen out of 20 (65%) had dry cavity at the end of 3 months. Of the 7 discharging cavity 3 were sterile and 4 showed growth on culture. The difference was non significant between both the groups (p>0.05).
Antibacterial activity of manuka honey was assessed by comparing preoperative aural swab and postoperative aural swab result at 3 months. We found an overall decrease in infection rate from 75% to 35% after 3 months in the study group. Eight out of 20 patients (40%) who had positive culture in preoperative sample went on to become sterile after 3 months (Fig. 2). Overall 13 out of 20 (65%) had sterile culture and 7 patients continued to show growth at 3 months. Manuka honey exhibited antibacterial activity against organisms like Pseudomonas, Proteus, Klebsiella, Escherichia, Staphylococcus aureus, etc., as shown in Table 2. Five patients had persistent infection by Pseudomonas after 3 months and 2 patients had persisting Proteus infection. Anaerobic infection was eradicated in all 6 patients by manuka honey (Table 2).
DiscussionMicrobial contamination of postoperative mastoid cavity is an important factor influencing healing of the cavity [1,13]. High concentration of antibiotics in serum doesnвҖҷt provide adequate bioavailability at the site of microbial contamination in the ear [5]. There is also a problem of antibiotic resistance [14]. There is continuous search for adjuvant medical products that would reduce the need for antibiotics in mastoid surgery. Manuka honey (medical grade) is one such alternative which has been found to be safe for application in ear and has antibacterial and antifungal properties [9]. It has high sugar content and low pH. It contains methyl glyoxal, D-glucono-Оҙ-lactone, hydrogen peroxide which is responsible for its antibacterial action. Low water content makes honey inhabitable to microbes. Manuka honey is also known to inhibit biofilm formation [15]. Henatsch, et al. [8] in their study concluded that the topical use of medical honey is an alternative treatment for patients with therapy-resistant discharge from open mastoid cavity.
We have studied the antibacterial role of manuka honey in the healing of postoperative mastoid cavity in absence of use of systemic antibiotics. In this study we found that manuka honey caused slight increase in postoperative otorrhea on day 8 and 1 month after surgery. However the otorrhea decreased and at the end of 3 months the otorrhea decreased significantly. This difference could be explained by the fact that honey being a hyperosmolar solution, draws water from the wound bed and this might explain the increased otorrhea seen in the initial postoperative period in manuka honey group. Henatsch, et al. [8] also reported a decrease in otorrhea post treatment with honey in their study.
Itching and discomfort score were also found to slightly more on day 8 and 1 month in the study group when compared to the control group. At the end of 3 months, most of patients had no complaints of itching or discomfort in the ears. We also did not notice any incidence of facial palsy with usage of manuka honey. We conclude that manuka honey is safe for topical application in the ear and does not cause additional pain, itching or discomfort in ear.
We analysed the postoperative culture results at day 8, 1st month, 2nd month, and 3rd month in both the groups. On comparing culture results between day 8 and preoperative aural culture results in the study group, we found that out of 15 patients who had growth on culture preoperatively, 13 of them continued to have growth and 2 became sterile. Three patients had additional anaerobic infection. There was a slight increase in the infection rate. This could be due to fact that honey being a hyperosmolar solution, draws water from the wound bed and could have led to more super added microbial infections.
While comparison of culture results at 3 months in the study group showed that 8 out of 15 patients who had growth in preoperative aural swab in manuka honey group became sterile and 7 patients continued to have positive culture of which 3 had same organism and 4 had different organism cultured. The rate of postoperative mastoid cavity infection decreased from 75% to 35%.
Anaerobic infection was present in preoperative aural swab in 3 patients. Six patients developed anaerobic infection on day 8. There was slight increase in the incidence of anaerobic infection on day 8. However manuka honey was able to eradicate anaerobic growth in all 6 patients at the end of 2 months. Four patients had negative anaerobic cultures by the end of 1st month and the remaining two patients became culture free at the end of 2 months. Manuka honey was shown to have anaerobic activity in our study.
Manuka honey exhibited antibacterial activity against organisms like Pseudomonas aeruginosa, Proteus mirabilis, Klebsiella pneumoniae, Escherichia coli, Staphylococccus aureus, etc. Pseudomonas was cleared from the mastoid cavity in 10 out of 15 (66.6%) patients, Proteus mirabilis in 4 out of 6 (66.6%), Klebsiella in 3 out of 3 (100%), Staph aureus in 1 out of 1, and E coli in 2 out of 2. Only gram negative organisms persisted beyond 3 months. Five patients had persisting infection by Pseudomonas and 2 by Proteus.
Ten out of 20 patients (50%) had dry cavity at the end of 3 months. Of the 10 discharging cavity, 5 was sterile and only 5 showed growth on culture. Persistent infection in the mastoid cavity is an important factor that influences the healing of mastoid cavity. The antibacterial and anaerobic action of manuka honey was found to be effective in achieving dry ear. The healing of mastoid cavity and results of sterile culture at 3 months showed that manuka honey could be used as alternative antibiotic preparation with good anti-Pseudomonal activity. We also did not notice any adverse reactions with use of manuka honey in ear and no incidence of facial palsy was reported with manuka honey.
Small sample size is a limitation of the study but this preliminary study does show that manuka honey can be used safely as alternative antibiotic preparation in ear surgeries in the era of antibiotic resistance.
NotesAuthor Contribution Conceptualization: Roshan Kumar Verma. Data curation: Niveditha Damodaran. Formal analysis: Niveditha Damodaran, Roshan Kumar Verma. Investigation: Archana Angrup. Methodology: Roshan Kumar Verma. Project administration: Roshan Kumar Verma. Resources: Niveditha Damodaran. Supervision: Roshan Kumar Verma, Naresh K Panda. Validation: Roshan Kumar Verma. Visualization: Roshan Kumar Verma. WritingвҖ”original draft: Niveditha Damodaran. WritingвҖ”review & editing: Roshan Kumar Verma, Naresh K Panda, Jaimanti Bakshi. Fig.В 2.Trends in mean itching, otorrhoea, discomfort, and pain at various follow-ups in manuka honey group. VAS: Visual Analogue Scale. 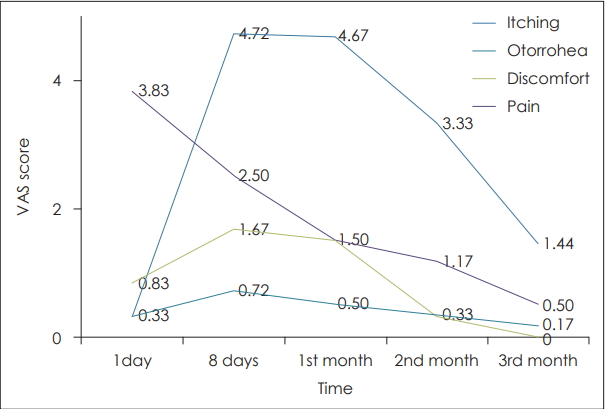
TableВ 1.Adapted mean merchant score at various times of follow up
TableВ 2.The difference in the clearance of infection of various organisms between manuka honey group and topical antibiotic group REFERENCES1. Mishra SG, Kushwaha JL, Yrat S. Microbial studies of post-operative mastoid cavities. Indian J Otolaryngol Head Neck Surg 1990;42(2):63-5.
2. Albert RR, Job A, Kuruvilla G, Joseph R, Brahmadathan KN, John A. Outcome of bacterial culture from mastoid granulations: Is it relevant in chronic ear disease? J Laryngol Otol 2005;119(10):774-8.
3. Macfadyen CA, Acuin JM, Gamble CL. Systemic antibiotics versus topical treatments for chronically discharging ears with underlying eardrum perforations. Cochrane Database Syst Rev 2006;(1):CD005608.
4. Carlin WV, Lesser TH, John DG, Fielder C, Carrick DG, Thomas PL, et al. Systemic antibiotic prophylaxis and reconstructive ear surgery. Clin Otolaryngol Allied Sci 1987;12(6):441-6.
5. Saunders JE, Raju RP, Boone JL, Hales NW, Berryhill WE. Antibiotic resistance and otomycosis in the draining ear: Culture results by diagnosis. Am J Otolaryngol 2011;32(6):470-6.
6. Winerman I, Segal S, Man A. Effectiveness of prophylactic antibiotic treatment in mastoid surgery. Am J Otol 1981;3(1):65-7.
7. Marais J, Rutka JA. Ototoxicity and topical eardrops. Clin Otolaryngol Allied Sci 1998;23(4):360-7.
8. Henatsch D, Wesseling F, BriedГ© JJ, Stokroos RJ. Treatment of chronically infected open mastoid cavities with medical honey: A randomized controlled trial. Otol Neurotol 2015;36(5):782-7.
9. Patel S, Cichello S. Manuka honey: An emerging natural food with medicinal use. Nat Prod Bioprospect 2013;3(4):121-8.
10. Kwakman PH, Te Velde AA, de Boer L, Vandenbroucke-Grauls CM, Zaat SA. Two major medicinal honeys have different mechanisms of bactericidal activity. PLoS One 2011;6(3):e17709.
11. Jenkins R, Burton N, Cooper R. Manuka honey inhibits cell division in methicillin-resistant Staphylococcus aureus. J Antimicrob Chemother 2011;66(11):2536-42.
12. Campeau ME, Patel R. Antibiofilm activity of Manuka honey in combination with antibiotics. Int J Bacteriol 2014;2014:795281.
13. Youngs R. The histopathology of mastoidectomy cavities, with particular reference to persistent disease leading to chronic otorrhoea. Clin Otolaryngol Allied Sci 1992;17(6):505-10.
|
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||

 |
 |